Latest News
Abcalis has been awarded with the ECEAE Prize for animalfree antibodies. Our co-founder Dr. Esther Wenzel gladly represented us at the prize ceremony in Brussels.
Abcalis participated in a worldwide study on the efficacy of SARS-CoV-2 cross-vaccinations
Abcalis' antibody against anti-SARS-CoV-2 Spike Protein S1 (RBD) binds to Omicron variant

Antibody generation & production
Selected via phage display

Affinity maturation
Improved affinity and binding characteristics

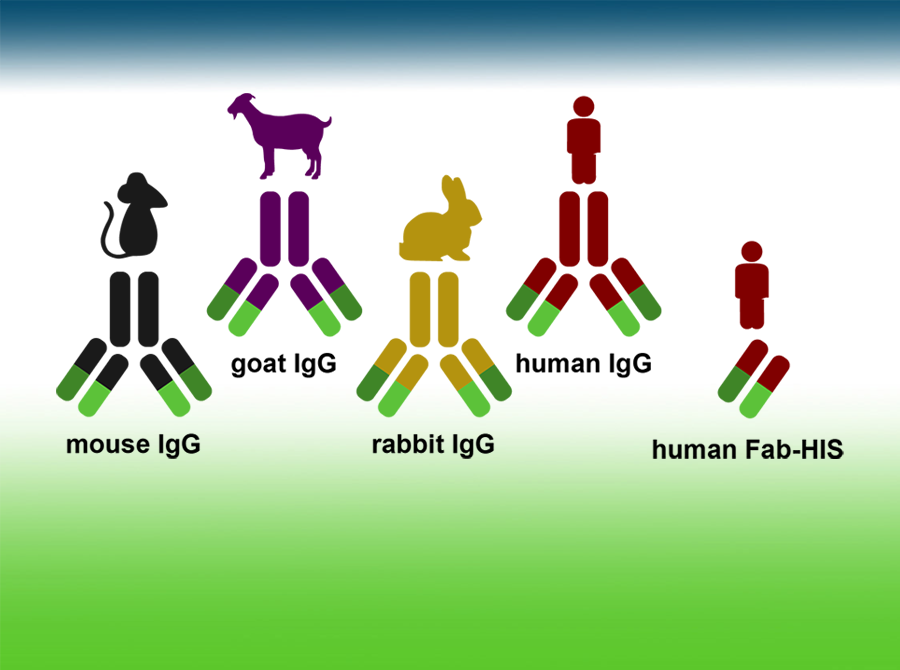
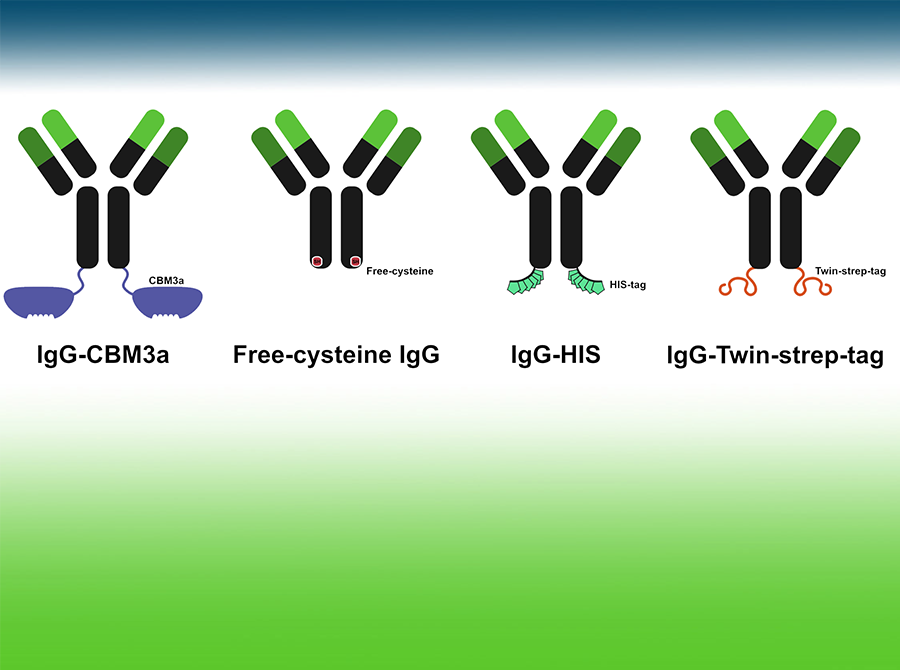
Antibody format conversion
Host species of choice, Fc-engineering, protein tag fusion

Naïve library generation
Representation of the complete resting state antibody repertoire
SERVICESWe find the best suitable Reagents for You
Tailor-made antibody services guarantee full
versatility and performance for your setup.
versatility and performance for your setup.


PRODUCTSHighly specific and sequence defined recombinant antibodies

BENEFITSStay competitive
Abcalis® vegan antibodies and services help diagnostic companies and researchers worldwide improve the quality, reliability and continuity of their tests and experiments.





ADVANTAGESMaking full use of sequence defined reagents
All Abcalis® antibodies are sequence defined and thereby truly immortalized in the form of digital data. Even if all biological samples are lost, the antibodies can still be recreated.
New level of product quality
Animal-free and sustainable
Scalable batch sizes
Unlimited reproducibility
Lower risk and QC costs
Regulatory future-proof


MISSIONOver 30 Years of Experience in Phage Display Technology
Our mission is to ensure antibodies sourced from animals will become a practice of past times.
Multi-generational libraries
High-quality results
Led by expert specialists
Nobel Prize technology